Electrochemical cells for energy conversion, gas cleaning and oxygen sensing
Efficient conversion of energy from electricity to storable fuel and back will play an increasingly important role for the energy infrastructure as the use of renewables like wind power continues to grow.
Electrochemical cells are ideally suited for this: As electrolysers they may utilize electricity to produce hydrogen or syngas which may be stored or processed into transportation fuels; as fuel cells they convert the chemical energy of the fuel into electricity with a high efficiency.
Solid oxide cells with an operation temperature of 500-1000 °C have also a potential as gas cleaning devices for both (conventional) power plants and for automotive uses. In addition, cells of the same type were already commercialized some decades ago as oxygen sensors in the form of lambda sensors for the automotive industry and special (expensive) oxygen sensors for other applications.
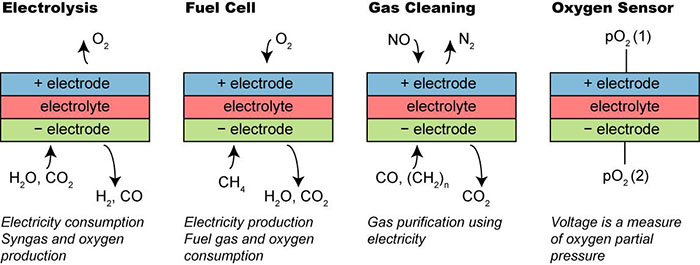
All four technology applications have been demonstrated but hitherto the cost has been too high for a commercial breakthrough to occur. To realise the enormous potential of the technology to the full, it is imperative to bring down the cost, i.e. to improve the performance and durability of the materials and components which enter into the cells.
Challenges of the electrodes
Although solid oxide fuel cells are coming closer to commercialization there are still fundamental materials challenges before a widespread use of the technology can be expected. These challenges are above all related to the electrodes where the electrochemical processes occur.
Good electrodes usually consist of two immiscible solid phases in a porous layer. Establishment of the relation between the performance (electrode polarization resistance) and the main electrode parameters – such as the physical properties of the given materials, volume ratio between the phases, the porosity, the coverage of electrode material on the electrolyte, the pore and particle size structure, and level of important impurities – will allow us to make a much better optimization. In state-of-the-art cells the main loss is in the electrodes even though theoretical considerations indicate that there should not necessarily be a large contribution to the losses from the electrodes.
One of the reasons for the unexpectedly high polarization resistance of both electrodes seems to be the partial blockage for charge transfer formed by segregation of impurities, e.g. in solid oxide cells SiO2 will form mono-layers with clear impeding effects for ion transfer even on the 10 ppm level. “Dissolving” suitable additives of transition metal oxides into the mono-layer may lower the resistance for oxide ion migration significantly.
Thus, part of the work will be in-situ studies of surface properties as a function of composition and surface topography.
Three phase boundary influence performance
All types of electrochemical gas cells are dependent on a long TPB (three phase boundary) at which the gas phase, the electron conducting electrode and the ion conducting electrolyte are in direct contact with each other. The reactions can only be fast where the three phases are in intimate contact so that the reactant concentrations are high, and the products can easily get transported away.
However, there is a strong tendency that the TPB gets partially blocked by segregation of impurities and/or cell constituents. Growth and agglomeration of the most active nanoparticles will often take place, decreasing the length of the TPB. Also, the detailed structure of the TPB region as well as the interplay of the materials properties are of great importance for both the performance and the durability of the cell. The knowledge about the surfaces and interfaces is very incomplete, and detailed knowledge about the TPB properties is close to non-existent.
The challenges
It is believed that solving the following problems will be of major importance for the achievement of widespread success for electrochemical cells:
- Development of new stable electrode materials with high electrocatalytic properties.
- Improvement of the electrical conduction of segregations to the interfaces and surfaces, and especially the TPB regions.
- Identification and development of an optimized nano-structured region of that part of the electrode which contacts the electrolyte; this includes:
3a) Development of electrochemical methods for a sufficiently detailed characterization of the surfaces and of the structure of the electrode-electrolyte interface region.
3b) Improving electron microscopic methods, especially preparation of samples for high resolution (1-10 nm) electron microscopy and development of fast quantitative image analysis methods to depict precise 3-dimensional structures.
3c) Establishment and experimental benchmarking of mathematical models describing the relations between the electrochemical performance, materials properties and the details of the composite structure.
SERC objectives
The research of the center is carried out through education of Ph.D. students and postdocs. The intention is that all Ph.D. students will be working at the place of a partner (other than their home institution) for a significant period of time.
The projects of the center are of the following types:
- Development of improved test facilities such as the CAHT-SPM (controlled atmosphere high temperature scanning probe microscopy) and pressurized test houses for cell testing.
- Development of improved synthesis methods for new as well as known materials;
- Characterization of the new electrode and electrolyte materials using new and detailed methods for characterization of especially surfaces and interfaces.
- Tests of electrodes and cells using new advanced analysis of electrochemical impedance spectroscopic data.
- Combined electrochemical and gas analysis.
- Development of software to do automatic quantitative image analysis of SEM micrographs of the complicated electrode structures.
- Development and experimental verification of accurate mathematical electrode models.